Electric vehicles, powered by renewable energy instead of finite fossil fuels, are the best green choice—but they could be more sustainable. As the most common power source in electric vehicles and portable electronics, lithium-ion batteries rely on an expensive and scarce metal to work. To spur more research into a more abundant and much cheaper alternative, magnesium, researchers in China assessed the current state of this research and offered potential paths forward to achieve more sustainable batteries.
They published their work on October 6 in Energy Material Advances.
“The development of cost effective and high performance batteries is in favor with the general public,” said paper author Xiu Song Zhao, professor at Institute of Materials for Energy and Environment, College of Materials Science and Engineering in Qingdao University. “With the rapid promotion of new energy vehicles, the demand for lithium-ion batteries is increasing, however, the resources of lithium on earth are scarce, and it is of great practical importance to develop and explore new energy storage technologies that can replace lithium-ion batteries.”
Lin explained that sodium-ion batteries for large-scale energy storage applications attract increasing attention, which would be a powerful candidate to substitute lithium-ion batteries.
“Sodium is much more abundant in the earth’s crust than lithium, making it more suitable for large-scale electrochemical energy storage. The potential of sodium-ion half batteries is 0.3 V higher than that of lithium-ion batteries, leading to a wider choice of electrolytes. And aluminum collectors can be used for the anode, which will again reduce the cost. However, the larger radius and heavier molar weight of sodium ion than lithium ion lead to fundamentally different requirements for electrode materials. Therefore, the development of high performance anode materials is one of the keys to realizing the sodium-ion batteries technology,” Zhao said.
“Titanium dioxide is a promising anode material for sodium-ion batteries owing to its non-toxicity, low cost, and the abundance of titanium in nature. Anatase is more conductive in storing sodium ions due to its special stacking of TiO6 octahedra with two-dimensional channels for Na+ transport.”
However, some obstacles hinder the research progress of anatase. According to Zhao, the key challenges for anatase include poor electron conductivity and ion diffusion that significantly limits rate capability and long-term cycling performance. The poor rate capability limits applications in high-power electronic devices, and poor cycling performance significantly hinders the practical realizations of sodium-ion batteries.
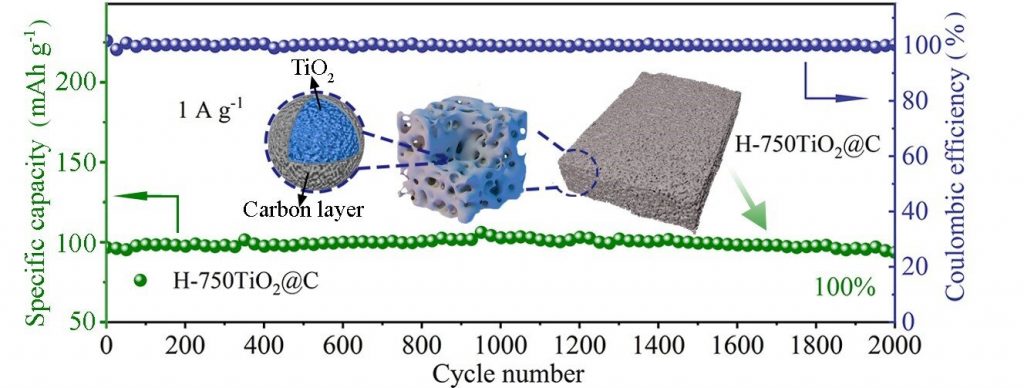
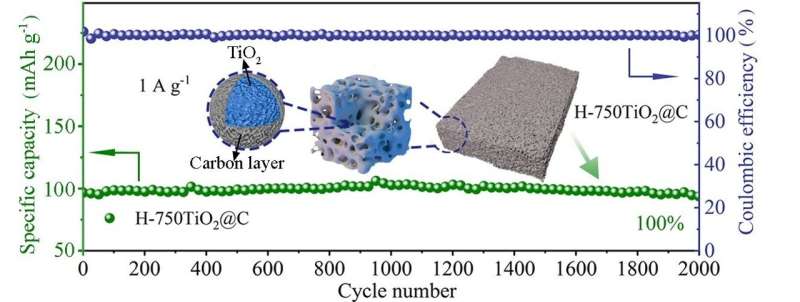
To overcome these challenges, Zhao said many studies focus on coating conductive materials, nano-structuring and constructing porous structures. Zhao and his team demonstrated a sol-gel method to synthesize a spongy, thermally stable anatase covered with carbon. The special structure creates channels for electrolyte ion transport and increases the interfacial area for charge storage.
When the synthesized anatase was tested in a half battery, the researchers found that the battery exhibited a reversible specific capacity of 228 mAh g-1 at a current density of 0.05 A g-1 with 100% capacity retention after 2,000 cycles at 1 A g-1. Both in-situ X-ray diffraction and Raman spectroscopy resulted reveal a nearly zero-strain characteristic of anatase during charge/discharge processes.
In-situ transmission electron microscopy, ex-situ X-ray photoelectron spectroscopy and scanning electron microscopy resulted suggest an irreversible sodiation-activation process to form a sodiated-TiO2 phase during the initial discharge process. A full coin cell assembled with anatase as the anode and Na3V2(PO4)3 as the cathode delivered an energy density of 220 Wh kg-1.
“This work prepared anatase with a special structure to improve the electron conductivity and ion diffusion kinetics, resulting in good rate performance and excellent cycling stability for sodium-ion storage,” Zhao said. “In-situ and ex-situ characterization reveals the sodium storage mechanism, indicating that a sodium activation process occurs during the initial sodiation resulting in a low initial coulombic efficiency. This work provides a method for synthesizing high performance titanium dioxide-based anode materials and ideas for studying the storage mechanism of the anatase.”